 Helicobacter pylori Test Kit (Dry Chemical Method)
Helicobacter pylori Test Kit (Dry Chemical Method)
This product is suitable for qualitative in vitro detection of urease produced by Helicobacter pylori in human tartar. Helicobacter pylori is one of the most common pathogens entering the digestive tract through the oral cavity, and it is the main pathogenic factor leading to chronic gastritis and peptic ulcer. The detection of urease in human tartar provides the basis for the auxiliary diagnosis of a Helicobacter pylori infection. At present, commonly used clinical detection methods include gastric mucosa biopsy and urea breath test, etc.
The Hp test strip in this product is coated with urea and phenol red, the urease produced by Helicobacter pylori in the human tartar sample can quickly catalyze the decomposition of urea into ammonia and carbon dioxide which leads to an increase in pH value and a characteristic color change of the phenol red in the test strip. Without the presence of urease in the tartar sample, no change of color will occur, indicating the absence of Helicoba
 Cytobot® Automated Circulating Tumor Cell Detection System
Cytobot® Automated Circulating Tumor Cell Detection System
Cytobot® 2000 is a CTC detection system completely independently developed by Huixian Pharmaceutical. It adopts the leading "physical + immune" microfluidic double enrichment principle, fully automatic operation, in-situ fluorescent staining, and has the characteristics of accuracy, automation, and efficiency.
 LunaDx® Nano Automatic Nucleic Acid Isothermal Amplification Analyzer
LunaDx® Nano Automatic Nucleic Acid Isothermal Amplification Analyzer
LunaDx® Nano automatic nucleic acid isothermal amplification analyzer is a portable nucleic acid amplification and detection analyzer based on LAMP technology. The instrument is small in size with extraction-free technology, and is easy to operate. The test results can be printed on site, which meets the needs of rapid nucleic acid detection in various scenes.
 LunaDx® Pro Fully Automated Nucleic Acid Detection Analyzer
LunaDx® Pro Fully Automated Nucleic Acid Detection Analyzer
Sample in, results out·Eliminate cross contamination
The fully automated nucleic acid detection analyzer is a closed platform integrating nucleic acid extraction, amplification, and fluorescence expression. The device is capable of testing for 52 pathogen nucleic acids in each sample within 2 hours, allowing for the rapid diagnosis of patients with clinically complicated infections.
The fully automated nucleic acid detection analyzer is based on PCR technology, offering high detection sensitivity. Stage heating and cooling allows for rapid temperature cycling, thus reducing the overall detection time. The fully closed chip design protects the environment and samples from cross-contamination. In a nutshell, the device makes nucleic acid detection more rapid, accurate,reliable and safe.
 LunaDx® Automatic Constant Temperature Nucleic Acid Amplification Analyzer
LunaDx® Automatic Constant Temperature Nucleic Acid Amplification Analyzer
Small, flexible and simple
The automatic constant temperature nucleic acid amplification analyzer is an automated, extraction-free nucleic acid amplification detection device based on LAMP technology, offering high detection sensitivity.
Through lightweight and miniaturization design, mobile grass-roots detection becomes more convenient.
Extraction-free technology reduces the dependence on field equipment and allows for easy handling.
The fully closed chip design protects the environment and samples from cross-contamination. In a nutshell, the device makes nucleic acid detection more rapid, simple, accurate, reliable and safe.
 Colorelief Methylated detection kit for Human Gene SDC2/SFRP2/TFPI2 (Multiple fluorescence PCR)
Colorelief Methylated detection kit for Human Gene SDC2/SFRP2/TFPI2 (Multiple fluorescence PCR)
Methylated DNA appears in the early stage of development of tumors. Holosensor developed ColoreliefTM Methylated detection kit for Human Gene SDC2/SFRP2/TFPI2 based on the new technology of Stool Methylated DNA detection. It helps physicians to detect colorectal cancer at its early stages when the malignancy can be effectively treated and stopped altogether.
 Magnetic Viral Nucleic Acid Extraction Kit
Magnetic Viral Nucleic Acid Extraction Kit
Extract nucleic acid (DNA, RNA) from swabs, sputum, respiratory lavage fluid, serum, plasma, environmental samples, water samples and other samples. The kit is composed of superparamagnetic nanospheres and an efficient extraction reagent system. The unique surface-modified magnetic beads have stronger nucleic acid binding ability and easier elution.
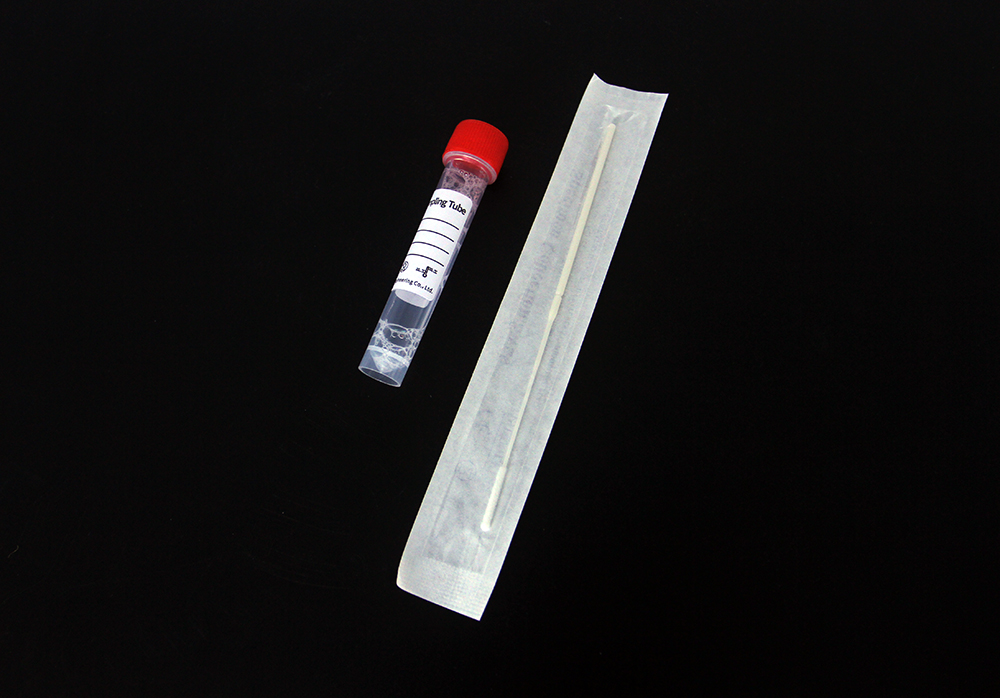 Pathogen Nucleic Acid Preservation Tube
Pathogen Nucleic Acid Preservation Tube
Inactivate DNase and RNase to prevent DNA/RNA degradation;
Inactivate viruses and other microorganisms;
Used for collection, transportation and storage of virus/pathogen samples,
such as nasopharynx, oropharynx, sputum, alveolar lavage fluid, etc.;
Sample stored at room temperature for 15 days;-20℃ for long-term storage.
 New Coronavirus COVID-19 Nucleic Acid Diagnostic Kit
New Coronavirus COVID-19 Nucleic Acid Diagnostic Kit
The primers and probes used in the diagnostic kit target the multiple regions both in open reading frame (ORF 1ab), Nucleocapsid protein (N) gene and Envelop (E) gene regions. It can fulfill the specific detection of both multi-regions and multi-genes which will reduce the risk of missed detection.
 CT+UU+NG Pathogenic Nucleic Acid Diagnostic Kit
CT+UU+NG Pathogenic Nucleic Acid Diagnostic Kit
Multiplex qPCR technology is used. Simultaneous detection of CT/UU/NG. Results are obtained within 60 minutes through single test. Greatly shorten the diagnosis time of pathogens with the same symptoms and improve diagnosis accuracy.